It's 1974: the first published peer-reviewed article claiming that chlorofluorocarbons (CFCs) are damaging the stratospheric ozone layer hits the news. CFCs, once used commonly in aerosol cans, fire extinguishers, refrigerators, and air conditioners, were responsible for causing depletion of the ozone layer. The paper created such a national stir that ozone depletion is still a major component of congressional scientific policy. So what is ozone? How do CFCs create the ozone hole? Is the ozone still there? Why should we care?
The Ozone Layer
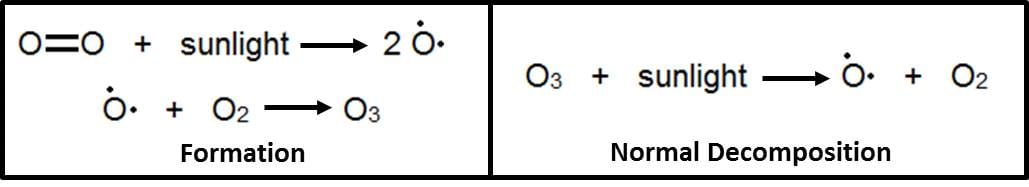
Ozone is a molecule in the Earth's atmosphere that impacts your daily life. Ozone is made up of three oxygen atoms (O3), and it is important to you because it protects your body from harmful ultraviolet (UV) rays from the sun. Ozone is produced in the atmosphere when molecular oxygen (O2) reacts with light from the sun, which causes it to split up into two oxygen atoms. These atoms then encounter another O2 and form ozone, O3.
When ozone absorbs UV light, it splits back up into one oxygen atom, and molecular oxygen. This new oxygen atom can now recombine with another O2 or another oxygen atom to make O3 or O2 respectively.
A Radical Chain Reaction
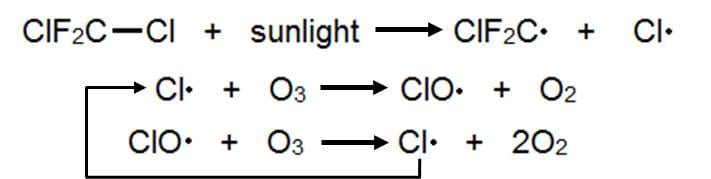
Like ozone, CFCs also absorb ultraviolet light, which causes a bond to break between a carbon and a chlorine atom. The reaction breaks off and sets loose a chlorine atom from the CFC that reacts with the ozone to form a new molecule, ClO (chlorine monoxide). ClO then reacts with another ozone molecule to regenerate the destructive Cl atom and form molecular oxygen.
This regeneration is the fundamental issue with the ozone hole. Not only does this cycle destroys two ozone molecules, meaning they can't absorb that UV light, but in the end the Cl atom is reproduced and goes on destroying more ozone in a chain reaction. Theoretically, one Cl atom could disrupt the entire ozone layer across the globe.
In practice, however, there are other atmospheric species that can react with the chlorine atom and trap it in more stable compounds, known as reservoir species, terminating the chain reaction. These reactions happen less frequently than ozone-destroying reactions, so on average one chlorine destroys over 100,000 ozone molecules before getting trapped.
Turning the Tide
The first reported detection of “the ozone hole†over Antarctica was in the journal Nature in 1985. However, ozone holes exist over both polar regions. These regions have extremely cold temperatures that facilitate clouds forming in the stratosphere. These clouds contain ice crystals that assist in freeing chlorine from the reservoir species. As a result, there is a local increase in ozone-depleting chlorine causing much larger decreases in atmospheric ozone as compared to other global regions.
In the late 80s, spurred by both scientific corroboration and several time-lapse videos of the ozone hole over the Antarctic, international legislation was passed to reduce CFC consumption to remediate the effects on the ozone layer. The bans sought to find alternative chemicals for industrial use that, if released, would not destroy the ozone in the stratosphere.
Over the last two decades, CFC consumption has dropped to near zero, replaced by hydrofluorocarbons (HFCs) that do not deplete ozone because the fluorine and hydrogen bonds of HFCs do not break as easily. Furthermore, these HFCs have a much shorter atmospheric lifetime than CFCs do.
False-color views of total ozone over the Antarctic pole from 1979 to 2015. The map shows purple and blue where there is the least ozone, and yellow and red are where there is the most ozone. You can follow the progress of the ozone hole by visiting NASA's Ozone Hole Watch page at http://ozonewatch.gsfc.nasa.gov/ Video: NASA Goddard Space Flight Center
Atmospheric lifetime is an average measure of the time a molecule will spend in the atmosphere before reacting. CFCs have a lifetime between 50-100 years, whereas HFCs have lifetimes less than 20 years. This means the HFCs have a significantly smaller chance of diffusing out of the troposphere, the layer of the atmosphere between the earth's surface and the stratosphere, where it is most probable these HFCs will be removed naturally.
These advantages and the world movement toward HFCs has had a marked effect on the ozone layer. The most recent World Meteorological Organization's Ozone Bulletin reports that the amount of ozone-depleting species is 9% less than its peak in 2002. Researchers at NASA's Goddard Space Flight Center recently published a projection that the ozone hole will remain larger than 8 million miles2, an area two and half times larger than the area of the 48 contiguous states, until 2040. The ozone hole grew from 38 thousand miles2 in 1979 to almost 11 million miles2 at its peak in 2006.
So should you worry about the ozone hole? Ozone depletion in an area smaller than 8 million miles2 is still very large. We should strive as a community to remove all traces of ozone depleting substances from the atmosphere. We have a long way to go, but it appears we have turned the tide on the ozone hole. Let's keep it up!
About the Author
 Joe Brice is a doctoral student in the University of Georgia’s Department of Chemistry, where he probes fundamental properties of bonding in small molecules of interest to astronomy, atmospheric or combustion chemistry. When not fiddling with lasers or stainless steel behemoths, he spends most of his free time (what free time?) fooling around with his fellow chemistry gluttons. Care to learn more? Sadly, Joe does not participate in this newfangled contraption known as social media. Try sending him an email, though, at joebrice@uga.edu! Joe Brice is a doctoral student in the University of Georgia’s Department of Chemistry, where he probes fundamental properties of bonding in small molecules of interest to astronomy, atmospheric or combustion chemistry. When not fiddling with lasers or stainless steel behemoths, he spends most of his free time (what free time?) fooling around with his fellow chemistry gluttons. Care to learn more? Sadly, Joe does not participate in this newfangled contraption known as social media. Try sending him an email, though, at joebrice@uga.edu! |
About the Author
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 17, 2020
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 12, 2020
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 3, 2020
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/March 30, 2020