Alexander Fleming's discovery of penicillin in 1928 has been heralded as one of the most monumental medical discoveries of all time. Amazingly, this discovery was entirely accidental. After returning from a long vacation, Fleming noticed that a culture of Staphylococcus aureus was contaminated with mold. After observing the culture under a microscope, he found that the mold, Penicillium, inhibited the growth of the bacteria. Fourteen years after its discovery, doctors used penicillin to save the life of a woman dying from a blood infection. Millions of lives have been saved since.
Before the existence of antibiotics, the immune system stood as the only defense against bacterial infections. At this time, an infection resulting from a small scratch could be as fatal as contracting pneumonia. Antibiotics have revolutionized medicine, reducing disease and mortality rates across the globe. Today, however, we face a dire crisis in the proliferation of antibiotic resistant bacteria as a result of antibiotic abuse and overuse. As the effectiveness of antibiotics wanes, scientific researchers need to identify new alternatives to prevent medicine from receding into the dark ages. Otherwise, we face returning to the days of untreated syphilis, the black plague, bacterial meningitis, typhoid fever, diphtheria, and tuberculosis.
When Alexander Fleming accepted the Nobel Prize in 1945 for his discovery of penicillin, he cautioned that the extensive use of antibiotics could result in bacterial resistance. His warning foreshadowed the crisis we now face today. One report projects that by 2050, 10 million people will die every year due to infections resulting from antibiotic resistant bacteria. See the image below for an explanation of how antibiotic resistance occurs and how it can spread through a population.
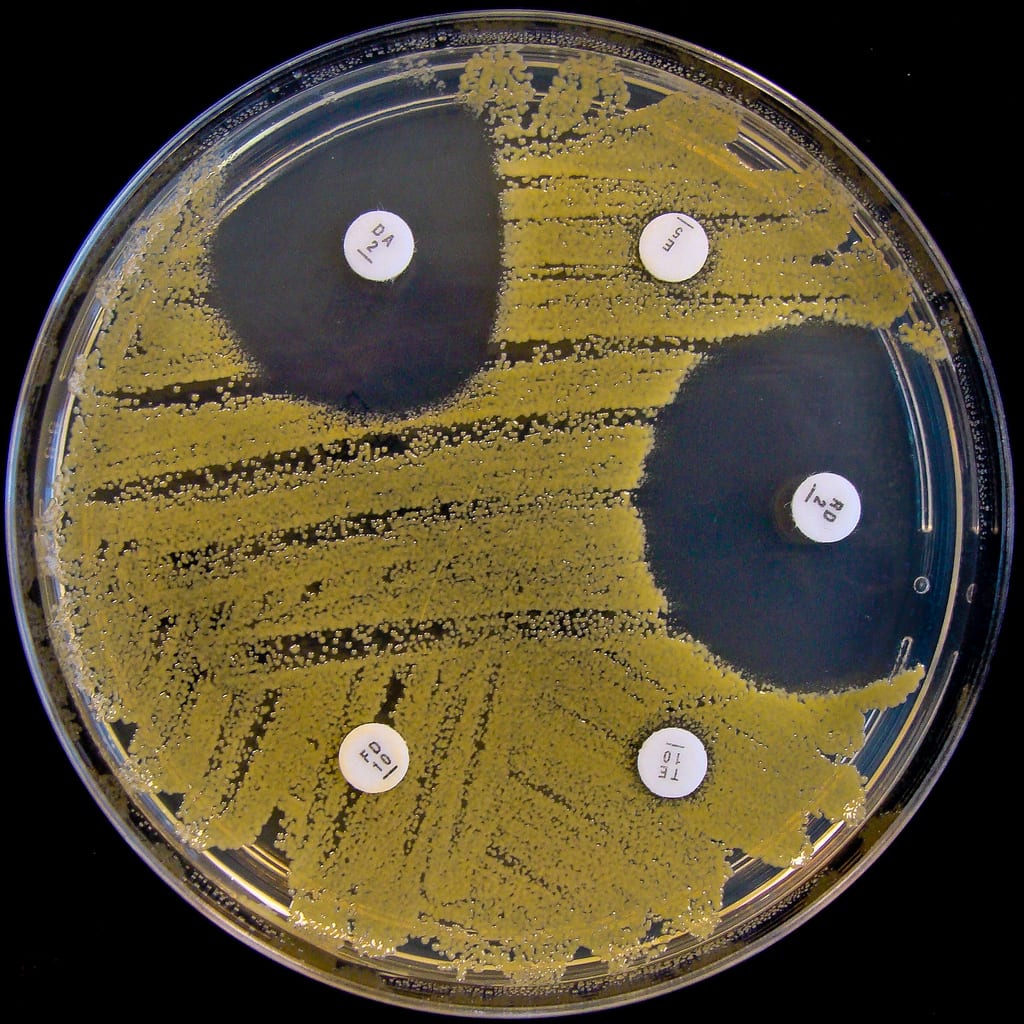
One of the most significant consequences of antibiotic overuse is MRSA, or Methicillin-Resistant Staphylococcus aureus. Although S. aureus is a common skin bacteria, overgrowth can cause a staph infection. Typical staph infections are easily cured with antibiotics. MRSA, on the other hand, is a multidrug-resistant staph infection. Its resistance to a wide range of antibiotics makes it extremely difficult to treat. Clinically, a MRSA infection usually begins as a swollen, inflamed, pus-filled skin lesion. In more severe cases, MRSA can infect the bloodstream or lungs, causing life-threatening illness. Vancomycin is one of the few antibiotics that can effectively target MRSA. Unfortunately, a clinical strain of vancomycin-resistant MRSA was reported in Japan in 1997. If this strain spreads, there may be no viable treatment options for infected patients.
The emergence of MRSA and other incidences of bacterial resistance have prompted funding for research to identify new sources of antibiotics from microbes that live in the soil. Standard laboratory practices for growing common bacteria like E. coli have been in place for many years, but growth requirements for the vast majority of organisms living in nature remain unknown. Without the ability to grow these environmental microbes, how can we determine whether they produce effective antibiotics? One method utilizes an instrument called the iChip. This device allows organisms to grow in their natural soil environment rather than in a laboratory, where these organisms are challenging, if not impossible, to grow. Each chamber of the iChip is inserted with a single soil bacterium and placed into the ground. Over two weeks, environmental nutrients diffuse into the chamber, promoting robust growth. In 2015, the iChip enabled the discovery of the antibiotic teixobactin.
As scientific research charges onward, the Small World Initiative (SWI) seeks to supplement the discovery of new antibiotics via crowdsourcing. SWI's program engages high school and college students to cultivate soil microorganisms in order to test their antibiotic properties against clinically relevant bacteria. If isolated soil microbes are found to inhibit bacterial growth, then the student has isolated a potential new antibiotic source. This initiative, while aiding the push for antibiotic discovery, also exposes students to the opportunities of STEM careers.
What will the future hold? Will we discover a series of novel antibiotics in the next decade to stave off multidrug-resistant bacteria? Or will a radically different approach, such as utilizing viruses to attack bacteria, hold the key to ending this crisis? The threat of widespread antibiotic resistance remains an urgent problem in desperate need of a solution, for the health and safety of this generation and the next. To contribute to the protection of yourself and your community, please practice smart antibiotic use!
About the Author
 Tina Ethridge is an undergraduate studying Applied Biotechnology with a minor in Genetics at the University of Georgia. In her free time, she enjoys hiking, cycling, and going on adventures with her retired racing Greyhound. Her favorite place to enjoy nature is at the top of Yonah Mountain in Helen, GA. Tina Ethridge is an undergraduate studying Applied Biotechnology with a minor in Genetics at the University of Georgia. In her free time, she enjoys hiking, cycling, and going on adventures with her retired racing Greyhound. Her favorite place to enjoy nature is at the top of Yonah Mountain in Helen, GA. |
About the Author
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 17, 2020
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 12, 2020
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 3, 2020
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/March 30, 2020