by: Stephanie M. Halmo
Researchers at North Carolina State University, Jagdish Narayan and Anagh Bhaumik, discovered a new solid phase of carbon distinct from graphite and diamond. Coined Q-carbon, this phase can quickly be converted to diamond at ambient temperature and pressure without the presence of hydrogen or a catalyst.
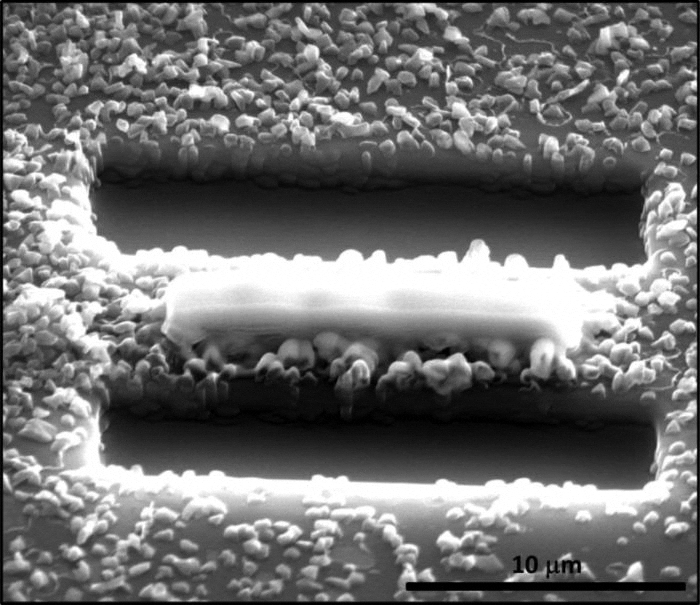
Before the discovery of Q-carbon, turning graphite into diamond required extremely high temperatures and pressure. Narayan and Bhaumik reported in the Journal of Applied Physics that Q-carbon can be used to form diamond structures, making production of the precious stone faster and easier.
To produce diamond structures, Narayan and Bhaumik melted carbon onto a sapphire substrate with a high-power laser. The carbon was cooled quickly, or quenched, to form Q-carbon. After subsequent laser pulses, Narayan and Bhaumik reported the creation of diamond structures, such as nano-needles and films, which can be used in industrial applications and electronics.
To determine the structure of Q-carbon, Narayan and Bhaumik measured atom vibrations by Raman spectroscopy. Compared to other solid phases of carbon, Q-carbon has enhanced electrical conductivity and is ferromagnetic.
 Stephanie Halmo is a former middle school science teacher turned graduate student, actively pursuing her Ph.D. in biochemistry from the University of Georgia. In her spare time she likes to dance, volunteer at local schools and tie-dye anything she can get her hands on. You can connect with Stephanie on Twitter and Instagram @shalmo or by email: shalmo27@uga.edu Stephanie Halmo is a former middle school science teacher turned graduate student, actively pursuing her Ph.D. in biochemistry from the University of Georgia. In her spare time she likes to dance, volunteer at local schools and tie-dye anything she can get her hands on. You can connect with Stephanie on Twitter and Instagram @shalmo or by email: shalmo27@uga.edu |