by Stephanie M. Halmo
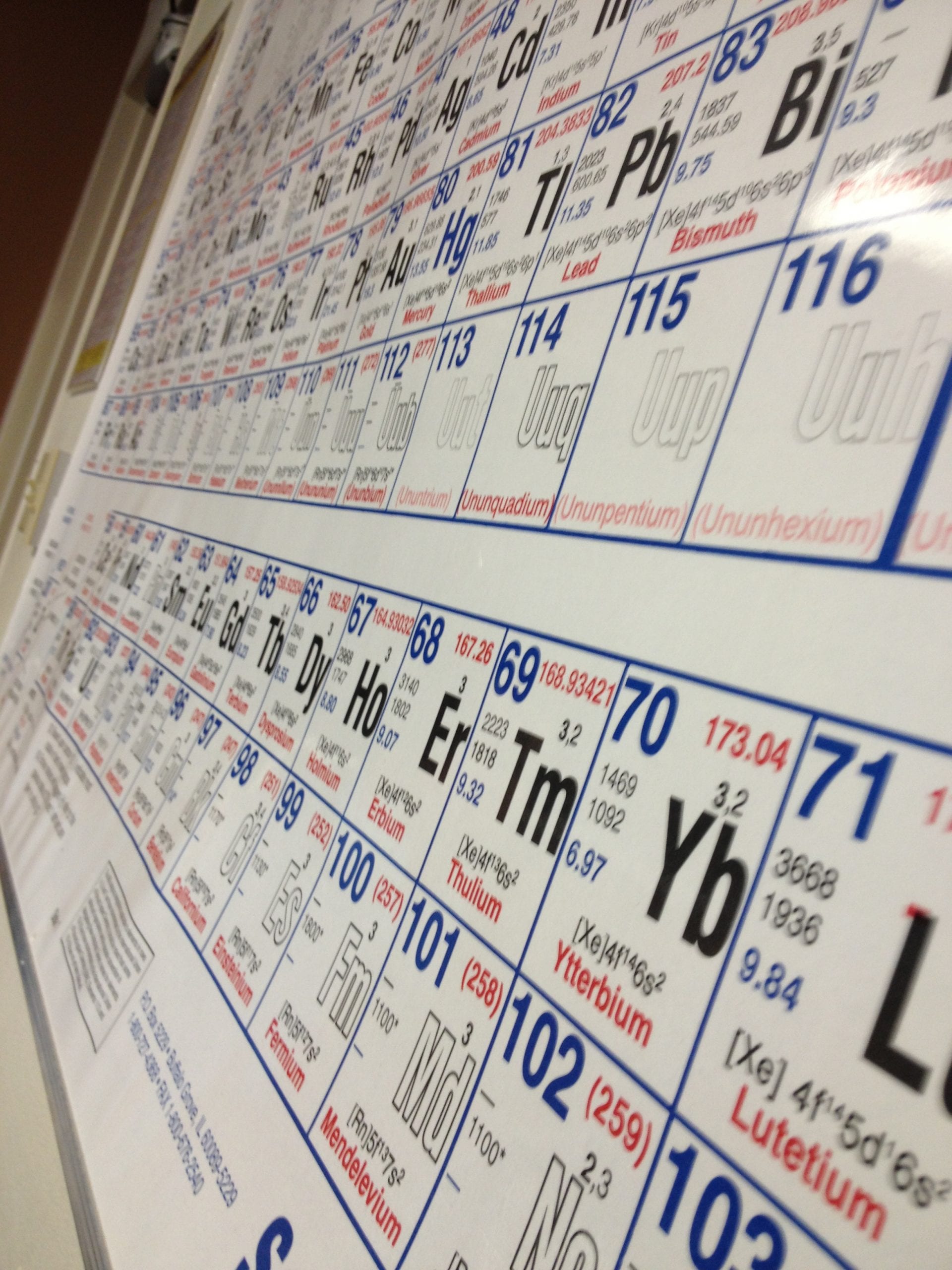
Scientists from across the globe have discovered four new elements, filling in row seven on the periodic table. The International Union of Pure and Applied Chemistry confirms the synthesis of these elements with atomic numbers 113, 115, 117 and 118.
The four new elements are not found in nature. Researchers synthesized the elements in a laboratory by bombarding super heavy atoms with beams of ions. The elements have been characterized as super heavy metals that are highly unstable, meaning they last only a fraction of a second before they decay. Due to this instability, scientists spent years gathering enough evidence to prove their existence.
Element 113 is the first element to be discovered in east Asia at the Riken Institute by Kosuke Morita's group. The discovery of the three other new elements – elements 115, 117, and 118 – are credited to a collaboration among the Lawrence Livermore National Laboratory in the United States, the Joint Institute for Nuclear Research in Russia and the Oak Ridge National Laboratory in the United States. The various institutes responsible for the discoveries now have the great honor of giving the elements their permanent names.
November 30, 2016 Update:
The International Union of Pure and Applied Chemistry announced the finalized names of the 4 new elements, and they are as follows:
Nihonium and symbol Nh, for the element 113,
Moscovium and symbol Mc, for the element 115,
Tennessine and symbol Ts, for the element 117, and
Oganesson and symbol Og, for the element 118.
 Stephanie Halmo is a former middle school science teacher turned graduate student, actively pursuing her Ph.D. in biochemistry from the University of Georgia. In her spare time she likes to dance, volunteer at local schools and tie-dye anything she can get her hands on. She is currently ASO’s News Editor. You can connect with Stephanie on Twitter and Instagram @shalmo or by email: shalmo27@uga.edu Stephanie Halmo is a former middle school science teacher turned graduate student, actively pursuing her Ph.D. in biochemistry from the University of Georgia. In her spare time she likes to dance, volunteer at local schools and tie-dye anything she can get her hands on. She is currently ASO’s News Editor. You can connect with Stephanie on Twitter and Instagram @shalmo or by email: shalmo27@uga.edu |



