Nature influences us every day. Not only can we find beauty in the natural world, but scientists and engineers can also draw inspiration from animals and plants to solve problems. Bioinspiration, a term for taking biological principles and applying them to non-biological science and technology, has long been a driving force behind invention and the development of new products.
Even the most unassuming creatures can spur advances in research. Take for instance Chrysomallon squamiferum, the scaly-foot snail. This diminutive gastropod, no more than 4cm across, has an incredible superpower. It is the only animal ever documented to be capable of creating its own built-in metal armor. The unique material used in its shell and scales has already inspired researchers to create new composite materials for a variety of applications.

While the scaly-foot snail gets its name from the shingle-like tiles that cover its body, both the scales and shell are made from a unique combination of metal and organic materials. All snails use a combination of hard minerals as well as soft organic matter to give their shells both strength and flexibility. The scaly-foot snail takes this even further by creating an additional outer layer of iron sulfide particles (yes, literal pieces of metal) embedded in organic material. In fact, there is so much iron in the snail's shell that they stick to magnets.
Why does the snail need the extra protection? One theory suggests that the snail developed the metal plating as a way to deal with its environment. Scaly-foot snails live near hydrothermal vents in the Indian Ocean. The super-heated eruptions from these vents are known to be acidic due to their high salt and sulfur content. Researchers found that the iron content made the snails' shells resistant to dissolving in acidic conditions. Additionally, sulfur is toxic to most marine organisms, so the snails could be depositing iron sulfides in their shells as a way to remove these toxic substances from their immediate surroundings.
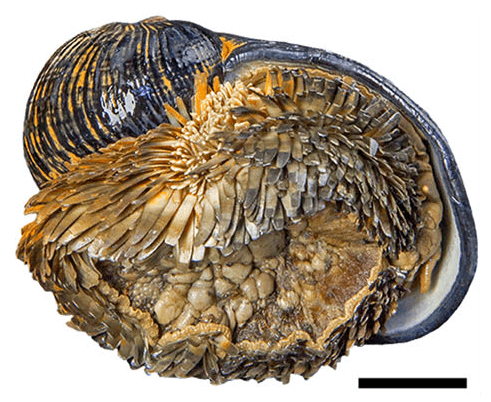
The second theory as to why the scaly snail needs a metal casing is that it provides protection from predators. If a hungry crab or carnivorous snail tries to puncture the iron-sulfide shell, a crack might start to form, but will be interrupted by the metal particles and spread in a different direction. This way, the shell can take on many tiny cracks, instead of a massive, fatal crack.
Furthermore, the shingled arrangement of the snail's namesake scales can act like a samurai warrior's armor, dissipating any single blow over many small sections. Lastly, the iron sulfide makes the surface of the snail especially hard, which may blunt a predator's claws or teeth. Our own tooth enamel is only about half as hard as the outer layer of the snail's scales. Ultimately, both theories speak to the snail's ability to survive in its environment.
For humans, there are many lessons to be learned from studying this unique creature. The most obvious application is to make armor ourselves. In fact, the U.S. military is currently developing body armor based on the shell structure of the snail. Sportswear, including helmets, may also be improved by taking the same approach. Perhaps a combination of metal particles in plastic would make lighter, more durable frames for cars and other transportation vehicles. Materials inspired by the scaly-foot snail might even be used in the international space station to reduce the risks of catastrophic cracks due to space debris.
Whatever the situation, we can look to nature for materials that have been developed over millions of years to fit their environment perfectly, and thank the scaly-foot snail for its exceptional shell and scales.
Featured image credit: Charlesjsharp via Wikimedia Commons
 Hailing from the deserts of Arizona, Mackenzie Carter is an enthusiastic masters student in the College of Veterinary Medicine. She is currently studying tissue engineering to model disease states in bone, namely panosteitis in canines. Mackenzie loves hands on projects, from ceramics to solar powered robots. In her free time she explores her passions: cephalopods, tea, and swing dancing. You can connect with Mackenzie via email at mackenziecarter@uga.edu.
Hailing from the deserts of Arizona, Mackenzie Carter is an enthusiastic masters student in the College of Veterinary Medicine. She is currently studying tissue engineering to model disease states in bone, namely panosteitis in canines. Mackenzie loves hands on projects, from ceramics to solar powered robots. In her free time she explores her passions: cephalopods, tea, and swing dancing. You can connect with Mackenzie via email at mackenziecarter@uga.edu.
About the Author
- athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 17, 2020
- athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 12, 2020
- athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 3, 2020
- athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/March 30, 2020