In 1963, at the dawn of the molecular biology age, Dr. Joshua Lederberg predicted that this nascent biotechnology would eventually allow direct control of human DNA, including the ability to change specific genes. [1] He recognized the potential of molecular biology to bring about gene therapy to cure human disease and to be misused for “genetic improvement†(i.e., eugenics), but his predictions were tempered by the acknowledgement that the techniques of his day were “pitifully clumsy.â€
“Surely within a few generations we can expect to learn tricks of immeasurable advantage,†he speculated.
In the decades following those lofty predictions, researchers have made great strides in the area of gene therapy. But for the most part, gene therapy remains a difficult, expensive, and unreliable approach to cure diseases.
That is, until now. We have—right now—the power to make changes to genes quickly, precisely, and cheaply. We have the potential to correct human diseases that arise from “typos†in the genetic code (e.g., cystic fibrosis). Theoretically, this type of gene therapy can correct errors in DNA in specific disease-affected tissues. This could also be used to edit the DNA of sperm or egg cells, in which case corrections can be inherited, potentially saving future generations from certain diseases.
This technology is called CRISPR [crisp·er ˈkris-pÉ™r](Figure 1). Its ease of use is what's brought us to the brink of developing the ultimate gene therapy tool—and what's holding us back from taking that next bold step. What are the long-term effects of human genome editing? CRISPR is precise, but how accurate is it? Could so-called off-target genes be changed unintentionally? Where do we draw the line between saving lives and tweaking this-or-that to “improve†the gene pool? How do we ensure that CRISPR is used for good and not for evil?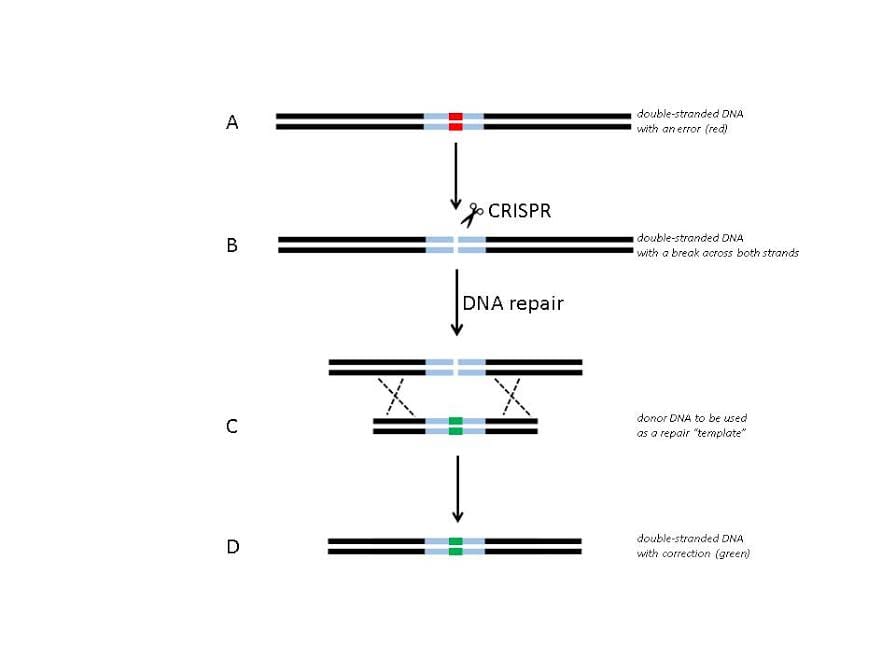 Figure 1. Theoretical gene therapy using CRISPR. (A) Imagine zooming in to view only a small portion of the genome that contains a gene (blue) with an error (red) in the DNA sequence. (B) Imagine programming CRISPR to locate this error and wrapping CRISPR in a package that can be delivered inside a cell. CRISPR finds its target and makes a double-stranded break where you programmed it to. The presence of this double-stranded break triggers the cell to repair the DNA. (C) If donor DNA is also provided in the package with CRISPR, it acts as a repair template that determines how the DNA will be repaired. The donor DNA could contain the gene with no error (green). (D) When DNA repair is complete, the gene in that cell is fixed! Figure was adapted from [2].
Figure 1. Theoretical gene therapy using CRISPR. (A) Imagine zooming in to view only a small portion of the genome that contains a gene (blue) with an error (red) in the DNA sequence. (B) Imagine programming CRISPR to locate this error and wrapping CRISPR in a package that can be delivered inside a cell. CRISPR finds its target and makes a double-stranded break where you programmed it to. The presence of this double-stranded break triggers the cell to repair the DNA. (C) If donor DNA is also provided in the package with CRISPR, it acts as a repair template that determines how the DNA will be repaired. The donor DNA could contain the gene with no error (green). (D) When DNA repair is complete, the gene in that cell is fixed! Figure was adapted from [2].
CRISPR: From Defending Bacteria to… Making Genetically Modified-Humans?!
CRISPR stands for “clustered regularly interspaced short palindromic repeats,†and it was discovered in bacteria. Normally, bacteria use CRISPR to defend against invasive pathogens (analogous to our immune systems). [3] Think of CRISPR as having two jobs: record-keeper and executioner. When a bacterium is invaded by a bacteriophage, it keeps a “record†of the invader by incorporating a snippet of bacteriophage DNA in its genome. If that bacteriophage comes around again, CRISPR recognizes the bacteriophage DNA and destroys the invader by chewing up its DNA.
The capacity to recognize specific DNA sequences and chew them up are the salient features of CRISPR that have been adapted by researchers to make precise changes to DNA sequences in an array of model organisms (such as plants, yeast, nematode worms, zebrafish, and mice). Notably, researchers have shown in human cells that latent HIV provirus can be disabled using CRISPR. Researchers have also used CRISPR to correct genetic defects in mouse models of human diseases, and to make changes to DNA in monkey embryos that give rise to genetically modified monkeys. Going from gene-modified monkey embryos to gene-modified human embryos would not require a huge technological leap.
But is CRISPR Ready for Prime Time?
The success of CRISPR to edit genes in model organisms and human cells raises ethical questions about its role in people. This month, at least three groups of prominent scientists urged caution and restraint in the use of CRISPR to modify the human genome in a way that can be inherited (i.e., in sperm, eggs, or embryos). [4-6] Many countries* ban or strictly regulate the modification of the human genome in this way, but many others do not. These public statements were meant to warn unscrupulous individuals against this type of research before the ramifications have been thought through. There is still debate even about the role of CRISPR in basic research—Is CRISPR okay to use in model organisms but not in humans? Is CRISPR okay in human somatic cells (not heritable) but not sperm or egg cells (heritable)? And so on.
At present, scientists advocate a moratorium on any clinical application of CRISPR and urge more open dialogue about CRISPR and its use in human genome editing. In addition, they recommend the creation of forums for education and discussion, evaluation of the utility of CRISPR in model organisms and in humans, and establishment of an international meeting of government agencies, ethics experts, and scientists to discuss issues and possibly make policy recommendations.
At the crux of all this lies an important issue that must be confronted: “What is the scenario that we're actually worried about? That [human genome editing using CRISPR] won't work well enough? Or that it will work too well?†If CRISPR works really well, then we could profoundly alter the course of human evolution.
*The US does not officially ban human genome editing, but the National Institutes of Health's “[Recombinant DNA Advisory Committee] will not at present entertain proposals for germ line alterations…â€
References:
- Lederberg J. Biological Future of Man. In: Wolstenholme G, ed. Man and his Future. London, England: J & A Churchill Ltd; 1963:263-273.
- Menke DB. Engineering subtle targeted mutations into the mouse genome. Genesis. 2013;51(9):605-618.
- Marraffini LA, Sontheimer EJ. CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat Rev Genet. 2010; 11(3): 181-190.
- Baltimore D, Berg P, Botchan M, et al. A prudent path forward for genomic engineering and germline gene modification. Science. 2015;DOI: 10.1126/science.aab1028.
- International Society for Stem Cell Research. The ISSCR Statement on Human Germline Genome Modification. Available at: http://www.isscr.org/home/about-us/news-press-releases/2015/2015/03/19/statement-on-human-germline-genome-modification. Accessed March 25, 2015.
- Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J. Don't edit the human germ line. Nature. 2015;519:410-411.
 Anna loves everything science, medicine, and art. When she's not at her day job as a medical writer, she can be found in her kitchen whipping up a sweet treat or outside trying to keep up with her critters. The perfect vacation for Anna would involve a state or national park, a museum, and yummy food.
Anna loves everything science, medicine, and art. When she's not at her day job as a medical writer, she can be found in her kitchen whipping up a sweet treat or outside trying to keep up with her critters. The perfect vacation for Anna would involve a state or national park, a museum, and yummy food.
About the Author
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 17, 2020
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 12, 2020
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/April 3, 2020
-
athenssciencecafehttps://athensscienceobserver.com/author/athenssciencecafe/March 30, 2020